ActinSimu
In silico study of actin-based force generation during cell movement or morphogenesis
Welcome to ActinSimu
The actin cytoskeleton plays a pivotal role in the generation of forces necessary for cell motility, morphogenesis or adhesion. In vivo, the actin filaments form networks resulting from their interplay with other protein partners [1]. However, the role of mechanical and/or geometrical constraints in the network organization is also important. Recently, we combined in vitro biomimetic system and patterning methods to constraint actin filament nucleation on patterns with specific geometry [2]. Using this technique, we have shown that geometrical factors, such as the distance between patterns or their shape, are responsible for the orientation of actin filaments, their interactions inside the network and transitions between networks with different organization [3]. To get a better understanding of how these geometrical constraints affect the network organization, we developed a piece of software dedicated to simulations of the cytoskeleton dynamics.
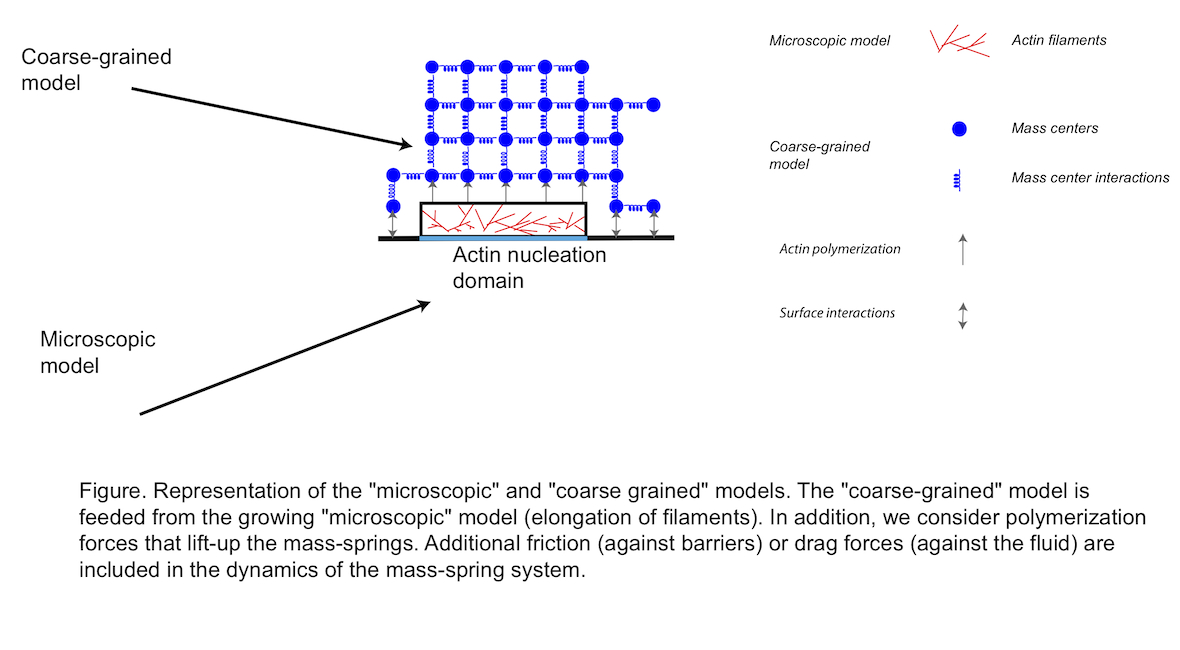
Our modeling approach consists into three phases. (i) First, we simulate the nucleation of actin filaments, their branching by the Arp2/3 complex and their elongation at a microscopic level [3]. In addition, we consider the effect of capping protein that binds to the barbed (active) end of actin filaments and stops their elongation. We also assume that filaments are short and straight. From now on, this first model is referred to as the “microscopic model” (Figure 1) and [4]. This “microscopic model” is exact but, for realistic concentration of Arp2/3 or actin monomers, simulations become rapidly cumbersome. In addition, it is difficult to answer simple questions about the mechanics at the level of the whole network. (ii) Therefore, to go further in the simulation of actin-based structures, we consider another model based on a mass-spring representation of the actin network. This second model is much easier to understand and more intuitive to simulate than the microscopic one. In addition, we can directly address questions about the mechanics or the force developed at the level of the whole actin structure. We refer to this part of the model as the “coarse-grained model” (Figure 1). (iii) The third and final step consist in the coupling between the two models, so that nucleated and elongated filaments inside the “microscopic model” are turned into the “coarse-grained” model and are used to update and/or generate node masses and springs (Figure 1
1. Pollard, T.D., and Borisy, G.G. (2003). Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453-465.
2. Reymann, A.C., Martiel, J.L., Cambier, T., Blanchoin, L., Boujemaa-Paterski, R., and Thery, M. (2010). Nucleation geometry governs ordered actin networks structures. Nat Mater 9, 827-832.
3. Achard, V., Martiel, J.L., Michelot, A., Guerin, C., Reymann, A.C., Blanchoin, L., and Boujemaa-Paterski, R. (2010). A "primer"-based mechanism underlies branched actin filament network formation and motility. Curr Biol 20, 423-428.
4. Kawska A., Carvalho K., Manzi J, Boujemaa-Paterski R., Blanchoin L., Martiel JL., Sykes C. (2012). How actin network dynamics control the onset of actin-based motility, Proc. Nat. Acad. Sci. USA 109,14440-14445
[ Back to top ]